Lithium metal batteries, in particular lithium-sulfur chemistries, have attracted a lot of attention as a next-generation energy storage technology that offers several advantages over conventional lithium-ion (Li-ion) batteries. Li-S batteries have a higher theoretical energy density, lower cost, and better environmental compatibility than Li-ion batteries.
However, one major concern with Li-S batteries is the environmental impact of finding, extracting, and transporting lithium, so using as little lithium as possible remains important. Moreover, Li-S batteries have certain limitations. Typically, these batteries consist of a lithium anode (negative electrode) and a sulfur cathode (positive electrode) with a separating layer. During charging and discharging, a large amount of lithium and sulfur react with each other, placing the lithium metal under a lot of strain.
To address these issues, researchers at Monash University have developed a new lithium-sulfur battery design that uses less lithium, has more energy per unit volume, lasts longer, and will be half the price of lithium-ion batteries.
To achieve this, researchers applied a nanoporous ultra glassy permselective Poly(trimethylsilyl)propyne polymer coating (PTMSP) directly to lithium foil. The thin polymer coating on lithium significantly improved the number of times the battery could be cycled.
The coating improves cycling performance through two mechanisms. First, the lowered LiPS permeability resulting from PTMSP’s discrete nanopores reduces capacity fading due to the irreversible loss of active sulfur and lithium via LiPS-anode reaction. Second, PTMSP acts as a scaffold to reduce the amount of high surface area mossy lithium formed during cycling and reduce the loss of lithium through dead lithium formation.
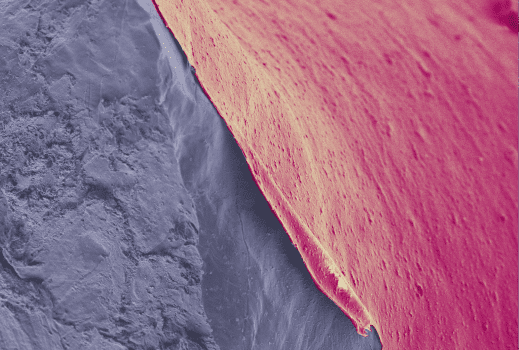
“The polymer contains tiny holes less than a nanometre in size – one billionth of a meter – which allows lithium ions to move freely while blocking other chemicals that would attack the lithium. The coating also acts as a scaffold for lithium and helps it charge and discharge repeatedly,” PhD student and lead researcher Declan McNamara of Monash Engineering said in the press release.
“Metallic lithium is a bit of a double-edged sword. Lithium is packed full of energy, but in a bad battery, this energy is wasted on side reactions. On the other hand, if the energy is channeled correctly, it can make some incredible energy storage devices that are easier to make. This coating is a step towards highly efficient, easily manufactured Li-S batteries,” Mr McNamara added.
In lab tests, the coated anodes exhibit 5.7 times more dense lithium over controls, translating to improved cycling performance due to increased capacity retention and improved lithium utilization.
In addition, the new design does not require nickel or cobalt, removing the need for minerals that have significant environmental and social costs.
These developments are promising steps for the more widespread adoption of lithium-sulfur batteries and other lithium metal-based energy storage systems.
“Li-metal protection technologies will become crucial in our quest towards energy-dense and sustainable batteries of the future. The study establishes a new framework to protect Li-metal from rapid decay or catastrophic failure, which has been an achilles heel for Li-S batteries,” he said.
