Popular metal-ion batteries (MIBs), such as lithium-ion batteries, are currently promising go-to energy storage solutions for makers of electric vehicles, phones, tablets, and laptops. However, these batteries suffer from environmental and economic issues because of their heavy dependency on non-renewable metals, threatening their long-term availability. Also, their toxicity and flammability can be unsafe and harmful to the environment.
Now, researchers at King Abdullah University of Science and Technology (KAUST) have shown rechargeable batteries that use ammonium cations as charge carriers could provide eco-friendly and sustainable substitutes to metal-ion-based batteries.
Previously, there have been several attempts to generate ammonium-ion-based batteries to solve sustainability and environmental issues because these cations are lightweight and easy to synthesize and recycle. However, ammonium cations are prone to reduction into hydrogen and ammonia at low operation potential, preventing the batteries from achieving their full potential. They also dissolve readily in electrolytes.
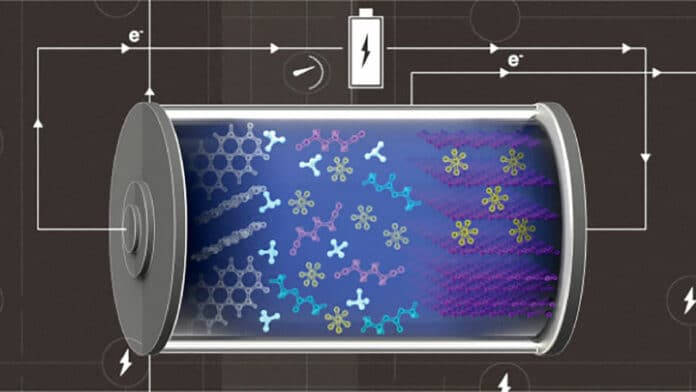
The KAUST team has developed a high-efficiency metal-free battery by combining an ammonium-cation-containing electrolyte with carbon-based electrodes. The graphite cathode and the organic semiconductor anode are cheap, environmentally friendly, and renewable, researchers say.
With the ammonium cations, they chose hexafluorophosphate ions as negative charge carriers and exploited the ability of graphite to reversibly accommodate these anions within its layers to create a “dual-ion” battery. In the battery, cations and anions simultaneously insert into their corresponding electrode during charge cycles and are released into the electrolyte during discharge cycles.
“We designed an electrolyte that is both antioxidative and anti-reductive by screening a series of solvents resistant to high voltage and also taking into account its reduction stability,” says postdoc Zhiming Zhao.
The antioxidative solvent mainly solvated anions participating in the cathode reaction, while its anti-reductive counterpart formed a solvation sphere around cations involved in the anode reaction. “This configuration is crucial for battery stability,” Zhao explains.
The battery outperformed existing ammonium-ion-based analogs with a record operation voltage of 2.75 volts. “It is now possible to develop high-energy nonmetallic ion batteries that can compete with metal-ion batteries,” Zhao says.
Researchers are currently working to enhance the performance to get closer to large-scale applications. “We are exploring anode materials with a higher capacity, which is crucial for improving the energy density,” Zhao says.
The team is developing cheap alternatives to lithium-ion batteries, particularly for grid-scale storage. “To eventually completely decarbonize the grid, the battery costs must significantly come down,” says Alshareef. Replacing lithium with nonmetallic charge carriers, such as ammonium ions, can help lower these costs.
Journal reference:
- Dr. Zhiming Zhao, Dr. Yongjiu Lei, Dr. Lin Shi, Zhengnan Tian, Dr. Mohamed N. Hedhili, Yusuf Khan, Prof. Dr. Husam N. Alshareef. A 2.75 V ammonium-based dual-ion battery. Angewandte Chemie International Edition, 2022; DOI: 10.1002/anie.202212941