Flow or redox flow battery technology can be a promising alternative for green electricity that requires large-scale storage to keep our power grids stable. Flow batteries store electricity in the tanks of liquid electrolytes that could be upsized to satisfy energy demands as they increase. However, these batteries contain rare metals and are expensive.
Scientists at the University of Groningen in the Netherlands have designed a flow battery electrolyte that may solve both problems.
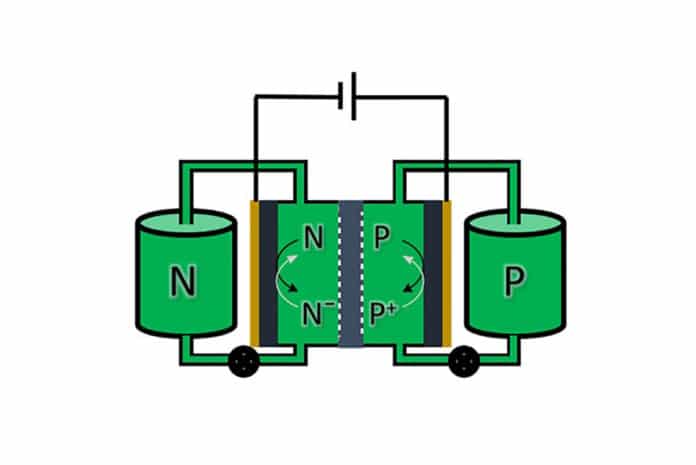
Since renewable energy is intermittent in nature, its application in power systems requires the use of large-scale storage. Using flow batteries is attractive because the problem can be tackled by storing energy in two separate fluids with dissolved chemicals in tanks for months at a time. In this case, chemical energy is converted into electricity when the liquid passes through a special membrane between the two tanks.
Traditional technologies use the scarce and expensive metal vanadium as an electrolyte source. Now University of Groningen researchers set out to design a new kind of flow battery material.
Both sides of the flow battery generally hold fluids with a different composition, but the scientists were able to develop a symmetrical design where both tanks contain the same fluid. Symmetrical batteries have been designed by linking the molecules that are used on both sides together and filling both tanks with the resulting hybrid molecule.
“The drawback of this approach is that only one part of the molecule is used on either side. And, during use, reactive radicals appear that degrade over time. This makes stability a problem,” says Edwin Otten, Associate Professor of Molecular Inorganic Chemistry at the University of Groningen.
Otten and his team looked for a single molecule that is stable, and that can accept or donate electrons and could, therefore, be used on both sides of the battery. The most promising compound was a Blatter radical, a bipolar organic compound with intrinsic stability. They tested the compound in a small electrochemical cell and found that it worked well and remained stable over 275 charge and discharge cycles.
“We need to bring this up to thousands of cycles; however, our experiments are a proof of concept. It is possible to make a symmetrical flow battery that has good stability,” Otten said.
The organic Blatter radical is relatively easy to make, and although it is currently not produced in industry, scale-up should be possible.
The next step is to create a water-soluble version of the Blatter radical, increase its stability and solubility, and test it on a larger scale. “The crucial test is to see whether our compounds will be stable enough for commercial applications,” said Otten, who led the research.