A team of engineers at the University of Illinois Chicago has built a cost-effective artificial leaf that can capture carbon dioxide at 100 times better than current technologies.
This novel artificial leaf works in the real world, unlike other carbon capture systems that could only work with carbon dioxide from pressurized tanks. It captures carbon dioxide from more dilutes sources, like air and flue gas produced by coal-fired power plants, and releases it for use as fuel and other materials.
“Our artificial leaf system can be deployed outside the lab, where it has the potential to play a significant role in reducing greenhouse gases in the atmosphere thanks to its high rate of carbon capture, relatively low cost, and moderate energy, even when compared to the best lab-based systems,” said Meenesh Singh, assistant professor of chemical engineering in the UIC College of Engineering and corresponding author on the paper.
The previous design proposed in 2019 consisted of a standard artificial photosynthesis unit that was encased in a transparent capsule made of a semi-permeable membrane of quaternary ammonium resin and filled with water. The membrane allowed water from inside to evaporate out when warmed by sunlight, and carbon dioxide was drawn in to replace it. The artificial photosynthetic unit inside the capsule converted the carbon dioxide to carbon monoxide, which can be captured and used to make synthetic fuels.
Now, engineers have modified a standard artificial leaf system with inexpensive materials to include a water gradient – a dry side and a wet side – across an electrically charged membrane. On the dry side, an organic solvent attaches to the captured carbon dioxide and turns it into a concentration of bicarbonate, or baking soda, on the membrane.
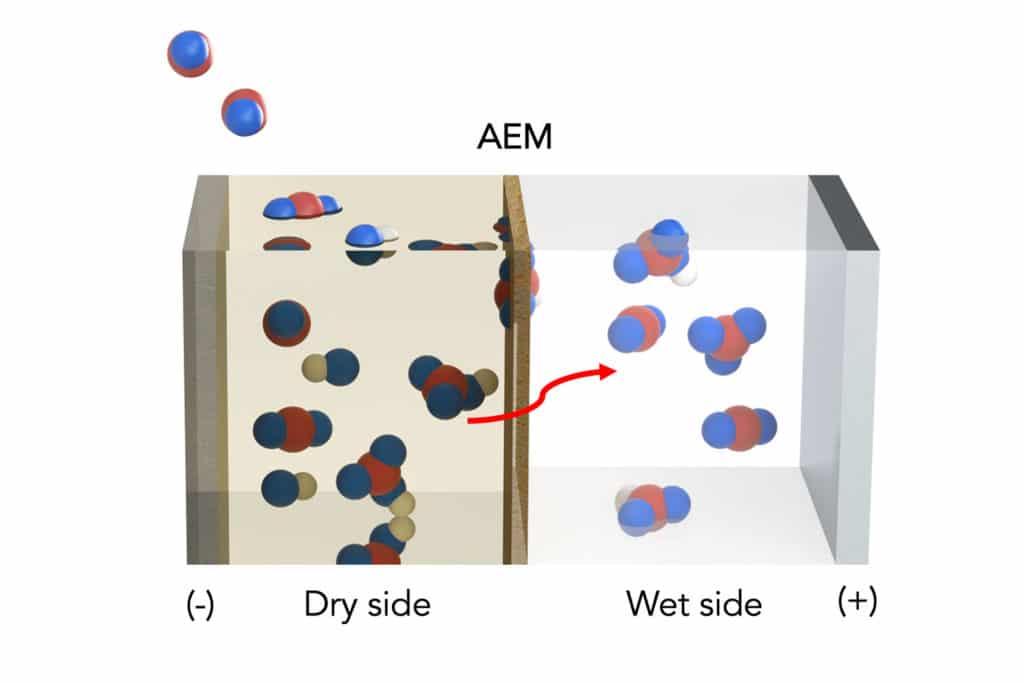
As bicarbonate builds, these negatively charged ions are pulled across the membrane toward a positively charged electrode in a water-based solution on the membrane’s wet side, where it is converted back into carbon dioxide to make fuels or in other applications. The electrical charge is used to speed up the transfer of bicarbonate across the membrane.
When they tested the system, the researchers found that it had a very high flux – a rate of carbon capture compared with the surface area required for the reactions. At its optimum, it could capture 3.3 millimoles per hour per 4 square centimeters, which is more than 100 times better than other systems. Importantly, only a moderate amount of electricity (0.4 KJ/hour) was needed to power the reaction, less than the amount of energy needed for a 1 watt LED lightbulb.
The team calculated the cost at $145 per ton of carbon dioxide, which is in line with recommendations from the Department of Energy that cost should not exceed around $200 per ton.
“It’s particularly exciting that this real-world application of an electrodialysis-driven artificial leaf had a high flux with a small, modular surface area,” Singh said. “This means that it has the potential to be stackable; the modules can be added or subtracted to more perfectly fit the need and affordably used in homes and classrooms, not just among profitable industrial organizations. A small module of the size of a home humidifier can remove greater than 1 kilogram of CO2 per day, and four industrial electrodialysis stacks can capture greater than 300 kilograms of CO2 per hour from flue gas.”