Traditional lithium-thionyl chloride (Li-SOCl2) batteries are widely used for their high energy density and other advantages. But unlike lithium ion or lithium polymer batteries, these cells cannot be recharged once they have been discharged.
A rechargeable lithium-chlorine (Li-Cl2) battery was first invented in 2021, with a high specific capacity of 1200 mAh/g, which is no less than six times greater than the 200 mAh achieved by today’s lithium-ion batteries.
However, a few problems stand in the way of the practical application of Li-Cl2 batteries. For example, the Cl2 reaction is limited by the weak physical adsorption of porous carbon to Cl2 molecules. Also, the excessive LiCl generation into the carbon pores blocks the channels for Li+ transportation and Cl2 diffusion, hindering further electrochemical reactions.
Moreover, the shuttle effect of unbonded Cl2 leads to battery capacity decay, especially at high output capacities. Therefore, cathode materials with highly porous structures are important for realizing high-performance Li-Cl2 batteries.
To overcome the above difficulties, a research team at the University of Science and Technology of China (USTC) adopted NH2-functionalized metal-organic frameworks (MOFs) in Li-Cl2 batteries to achieve high specific capacities, cycle stability, and superior low-temperature performance.
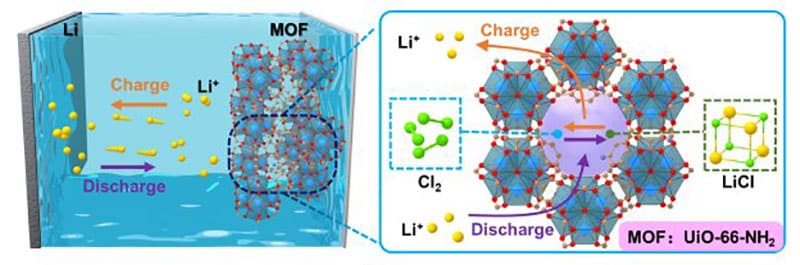
Predicted by theoretical calculations, highly porous MOFs with -NH2 functional groups, namely UiO-66-NH2, were screened out as a model and applied in Li-Cl2 batteries. The Li-Cl2 battery using NH2-functionalized MOF (Li-Cl2@MOF) demonstrated a maximum discharge specific capacity of 2000 mAh/g and is stable for more than 500 cycles under a specific capacity of 1,000 mAh/g at room temperature.
The batteries also performed stable functions under the temperature of -40 °C. This work presented an innovative design of cathode materials in Li-Cl2 batteries, paving the way for future applications of rechargeable Li-Cl2 batteries.
Journal reference:
- Yan Xu, Long Jiao, Jiale Ma, Pan Zhang, Yongfu Tang, Lingmei Liu, Ying Liu, Honghe Ding, Jifei Sun, Mingming Wang, Zhenyu Li, Hai-Long Jiang, Wei Chen. Metal-organic frameworks for nanoconfinement of chlorine in rechargeable lithium-chlorine batteries. Joule, 2023; DOI: 10.1016/j.joule.2023.02.010
