Due to their high theoretical energy density and low cost, lithium-sulfur batteries (LSBs) have attracted considerable attention as a potential alternative to conventional lithium-ion batteries.
However, LSBs face several obstacles that hinder their practical application, such as lithium polysulfide formation and dendrite growth during battery operation.
Now, a team of researchers from Japan and China has developed a battery separator consisting of platinum-doped atomically precise gold nanoclusters embedded into a graphene nanosheet. The resulting batteries demonstrate higher energy density, improved performance, and enhanced cycling stability.
Recently, atomically precise metal nanoclusters, aggregates of metal atoms ranging from 1-3 nanometers in size, have been attracting a lot of interest in materials research because of their high designability as well as unique geometric and electronic structures. However, the current research on metal nanoclusters is mainly fundamental, and their practical applications are still uncharted.
The research team, led by Professor Yuichi Negishi of Tokyo University of Science (TUS), has harnessed the surface binding property and redox activity of platinum (Pt)-doped gold (Au) nanoclusters, Au24Pt(PET)18 (PET: phenylethanethiolate, SCH2CH2Ph), as a high-efficiency electrocatalyst in LSBs.
The team prepared composites of Au24Pt(PET)18 and graphene (G) nanosheets with a large specific surface area, high porosity, and conductive network. These composites were used to create a battery separator that accelerates the electrochemical kinetics in the LSB.
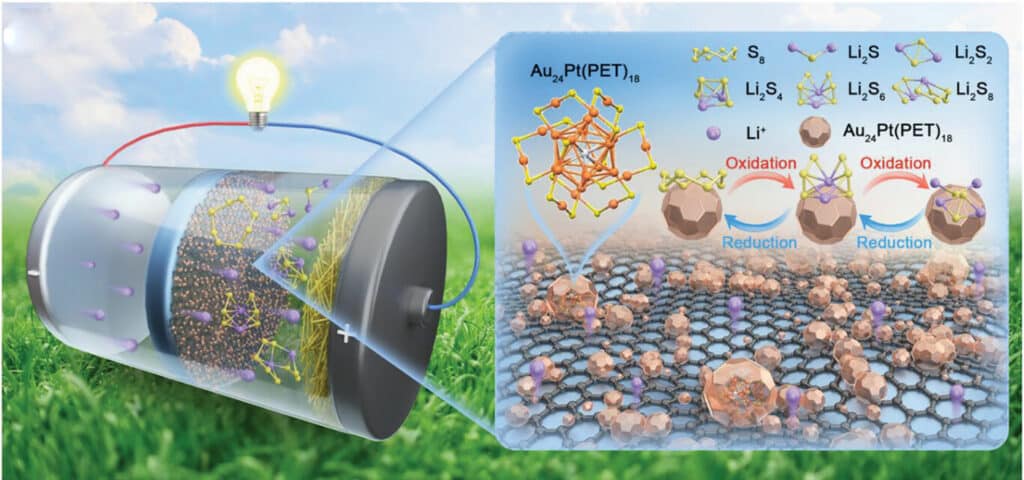
“The LSBs assembled using the Au24Pt(PET)18@G-based separator arrested the shuttling LiPSs, inhibited the formation of lithium dendrites, and improved sulfur utilization, demonstrating excellent capacity and cycling stability,” highlights Prof. Negishi.
Resultantly, the LSB using an Au24Pt(PET)18@G-based separator presents a high reversible specific capacity of 1535.4 mA h g−1 for the first cycle at 0.2 A g−1 and an exceptional rate capability of 887 mA h g−1 at 5 A g−1. Additionally, the capacity retained after 1000 cycles at 5 A g−1 was 558.5 mA h g−1.
This study is a significant step toward the application of metal nanoclusters as optimal electrocatalysts for LSBs and other sustainable energy storage systems. They can enhance the energy density, cycle life, safety, and environmental friendliness of LSBs, making them more environment-friendly and competitive with other energy storage technologies.
“LSBs with metal nanoclusters may find applications in electric vehicles, portable electronics, renewable energy storage, and other industries requiring advanced energy storage solutions. In addition, this study is expected to pave the way for all-solid-state LSBs with more novel functionalities,” highlights Prof. Negishi in an official statement.
