One of the primary devices for the treatment of more severe cases is the ventilator, which serves as an “electronic lung” for patients. As the traditional commercial ventilator available are expensive and require a high technical degree for assembly, companies from various sectors have been developing their own models to meet the high demand.
Fitbit, a company, known and appreciated for its health and wellness devices, has announced that it has developed a high-quality, low-cost, and easy-to-use emergency respirator, Fitbit Flow, to help address urgent global needs during COVID-19 crisis.
It has obtained an Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for use during the COVID-19 public health emergency. Although the product may soon reach the final consumer, distribution should initially be restricted to government agencies and to help health authorities around the world.
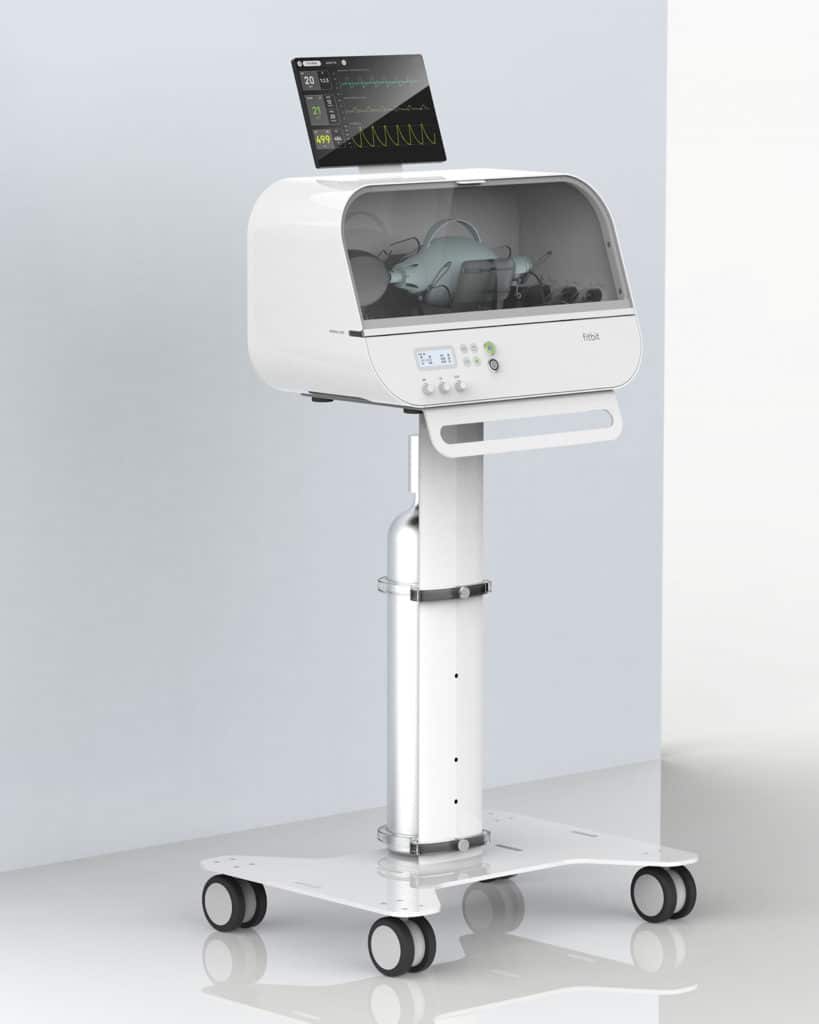
Credits: Fitbit
Fitbit Flow has a mixture of sophisticated instruments, advanced sensors, and alarms that work together to support automated compressions and patient monitoring. The device is designed to be intuitive and simple to use, potentially helping to reduce the strain on specialized staff who are typically needed to operate a commercial ventilator. It also meets “the same lower price range” as other emergency ventilators, says Fitbit. The novelty relies on the technology developed by the company in its activity tracking devices.
The company’s ultimate goal is to supply these devices to health care systems around the world that do not have a sufficient number of traditional commercial ventilators. Although the Fitbit Flow emergency ventilator is recommended by experts, the company itself suggests using it “when a traditional commercial ventilator is not available.”
There is no information on its costing and commercialization to the public. However, it is possible that its sale will soon be released to the population under medical monitoring guidelines.
