Carbon dioxide emission is considered one of the major causes of global warming. One possible solution is the electrochemical conversion of carbon dioxide into more useful molecules such as carbon monoxide, formic acid, and hydrocarbons – which are important intermediates in fuel and chemical production. However, the energy demand of the reaction is still too high.
Now, researchers from the University of Twente, in collaboration with Shell, have developed a new mechanism that makes the conversion of carbon dioxide into carbon monoxide, which is an essential feedstock in the production of chemicals.
“With the novel molecules designed in our research, we could innovate a new pathway for CO2 conversion,” UT Ph.D. student and lead author Sobhan Neyrizi said.
Developed by Sobhan Neyrizi and his fellow researchers, the new molecules can assist the electrochemical conversion of carbon dioxide. “Our molecules act as co-catalysts that reduce the energy demands of the reaction to a great extent,” says Neyrizi. The researchers argue they can use these molecules to innovate a new pathway for the reaction. “We could also propose design principles for the development of more efficient molecules in the future.”
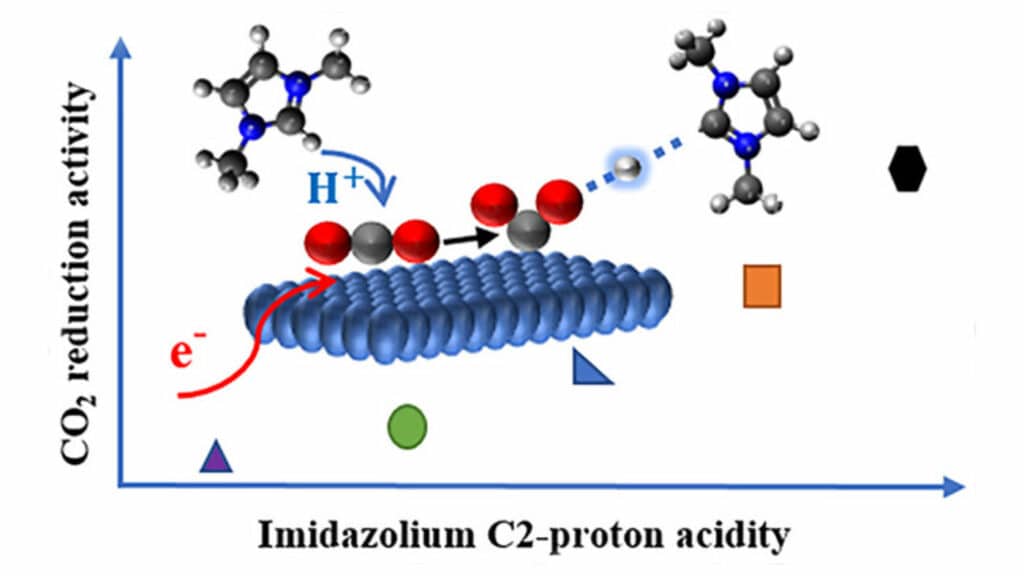
Electrons are a low-cost energy source in electrochemistry. The process requires the conversion of electrons to carbon dioxide, but this requires a lot of energy. The energy needed can be drastically reduced by simultaneously transferring protons and electrons to carbon dioxide molecules. The designed co-catalysts make this simultaneous transfer possible on a surface of gold.
“We could reach 100% efficiency for the conversion, which means that all electrons we put into the reaction get used”, explains Neyrizi.
The demonstrated mechanism provides guidelines for improvement in the energy efficiency of non-aqueous electrochemical CO2 reduction by a tailored design of electrolyte cations.
Journal reference:
- Sobhan Neyrizi, Joep Kiewiet, Mark A. Hempenius, and Guido Mul. What it takes for Imidazolium Cations to promote electrochemical reduction of CO2. CS Energy Lett. 2022, 7, 10, 3439–3446. DOI: 10.1021/acsenergylett.2c01372