Hydrogen is a promising future fuel, especially if produced from water. However, the noble metal catalysts required to make this work are rare, expensive, and have insufficient reserves. Also, they don’t perform as well as needed in harsh and acidic conditions that increase energy efficiency.
Now, researchers from the University of Tsukuba and collaborating partners have developed catalysts that can overcome the limitations of noble metals. Professor Yoshikazu Ito and co-workers use numerous non-noble metals all at once.
The team used high-entropy alloys, which are mixtures consisting of many elements. While some of these alloys can be used to generate large quantities of hydrogen, others undergo a process called oxidation which gives the alloys some corrosion resistance ability.
Nevertheless, as Professor Ito points out, “it’s apparent that identifying which metals to use in which proportions by conventional bottom-up experiments – i.e., change/add one metal at a time – is quite time-consuming. Our top-down approach not only saves time but also provides invaluable chemical insights into the hydrogen production mechanism.”
The team’s approach involves first uniformly mixing the nine elements that constitute the alloy, then passivating the surface in acidic media, and finally applying a voltage to facilitate surface structure rearrangements that optimize the activity of the catalyst.
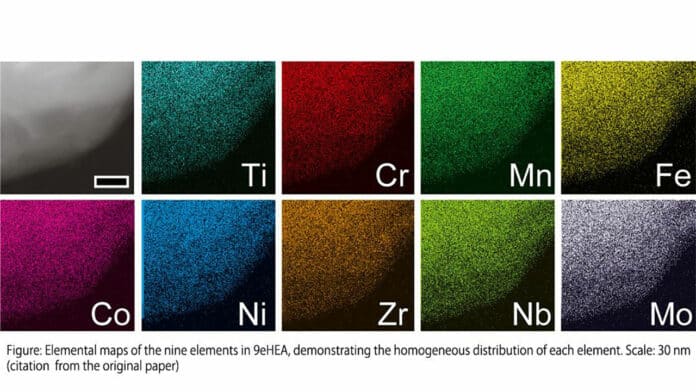
Experiments and theoretical calculations identified the metals that contributed to catalytic activity (e.g., iron, chromium, nickel, cobalt, and manganese) and the metals that contributed to passivation (e.g., titanium, zirconium, niobium, and molybdenum). The high-entropy alloys’ remarkable performance and corrosion resistance were demonstrated in practical water electrolysis experiments.
The study offers novel possibilities and new approaches toward replacing the highly scarce noble metals, especially iridium, with a worldwide production of only 7 tons per year. With the increase in the use of water electrolyzers globally, the demand for iridium is expected to be 700 kilograms per gigawatt.
“In the experimentally challenging conditions of 0.5 molars sulfuric acid electrolyte, it was necessary to sacrifice some stability to maximize the activity, and vice versa,” explains Ms. Aimi A. H. Tajuddin (1st author, Ph.D. student). “The catalyst exhibited an overvoltage of only hundreds of millivolts at 10 milliamps per square meter in the hydrogen and oxygen evolution reactions. Furthermore, its remarkable performance remained steady during electrochemical cycling tests, equivalent to 3-4 years of endurance in water electrolyzers operated by intermittent renewable energy sources such as solar power.”
The new research provides a proof-of-concept for a novel, noble-metal-free, economically feasible hydrogen production method from water by using renewable energy. The method costs only a few hundred U.S. dollars per kilogram of catalyst and is suitable for mass production. The application of high-energy alloys will allow for the production of hydrogen batteries and other products that would otherwise require expensive metals.
Journal reference:
- Aimi A. H. Tajuddin, Mitsuru Wakisaka, Tatsuhiko Ohto, Yue Yu, Haruki Fukushima, Hisanori Tanimoto, Xiaoguang Li, Yoshitatsu Misu, Samuel Jeong, Jun-ichi Fujita, Hirokazu Tada, Takeshi Fujita, Masaki Takeguchi, Kaori Takano, Koji Matsuoka, Yasushi Sato, and Yoshikazu Ito. Corrosion-resistant and high-entropic non-noble-metal electrodes for oxygen evolution in acidic media. Advanced Material, 2022; DOI: 10.1002/adma.202207466