The world is quickly moving into a new era of increased emphasis on green technologies. These technologies require more practical and accurate research and development (R&D) to meet the growing demands for energy storage systems. Recent advanced have led to more efficient lithium-ion batteries; yet, significant shortcomings remain. One of these challenges is the need for faster charging, which can help speed the adoption of electric vehicles.
Now, using the resources of the U.S. Department of Energy’s (DOE) Argonne National Laboratory, a research team led by Boise State University and the University of California San Diego has created a high-performance material for battery electrodes that could facilitate much faster charging for lithium batteries. It shows promise for speeding up charging while providing excellent storage capacity.
As lithium batteries cycle, lithium ions move from the positive electrode (cathode) to the negative electrode (anode), commonly made of graphite. At faster-charging speeds, lithium metal tends to accumulate on the graphite’s surface. Known as lithium plating, it compromises the battery’s performance and can cause it to short circuit, overheat or even catch fire.
Researchers sought to remove this roadblock on the route to faster charging with the help of a compound called niobium pentoxide, with a novel crystalline structure. Niobium pentoxide is much less susceptible to plating, potentially making it safer and more durable than graphite. Its atoms can be easily arranged into many stable configurations that don’t require much energy to reconfigure. This presents opportunities for researchers to discover new structures that could enhance battery performance.
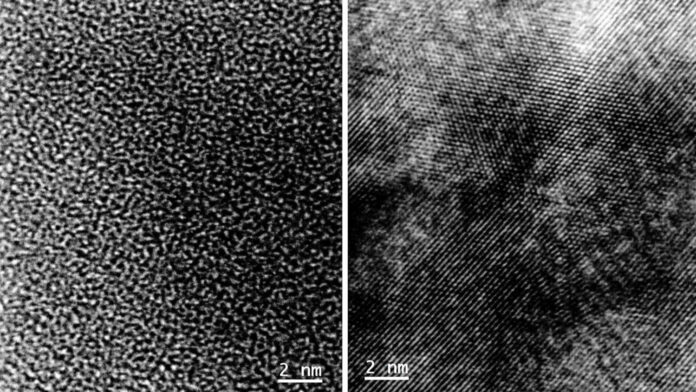
For their study, researchers built a coin cell with niobium pentoxide as the electrode material. Niobium pentoxide had a messy, disordered arrangement of atoms to begin with. But the team found that when the cell was charged and discharged several times, the disordered structure transformed into an ordered, crystalline one.
Described as a cubic rock-salt framework, the crystalline structure enabled easier, faster transport of lithium ions into the anode during charging. Their finding points to the material’s promise for fast charging, and other measurements suggest that it can store a large amount of charge. It exhibits superb cycling stability with a capacity of 225 mAh g−1 at 200 mA g−1 for 400 cycles and Coulombic efficiency of 99.93%.
It is very difficult to make high-performance, crystalline niobium pentoxide with traditional synthesis methods, such as those that subject materials to heat and pressure. The unconventional synthesis approach used successfully in this study – charging and discharging a battery cell – could be applied to make other innovative battery materials. It could potentially even support the fabrication of novel materials in other fields, such as semiconductors and catalysts.
Journal reference:
- Barnes, P., Zuo, Y., Dixon, K. et al. Electrochemically induced amorphous-to-rock-salt phase transformation in niobium oxide electrode for Li-ion batteries. Nat. Mater. 21, 795–803 (2022). DOI: 10.1038/s41563-022-01242-0