It is a well-known fact that pure, distilled water is an almost perfect insulator and does not conduct electricity. It consists of H2O molecules that are loosely linked to one another via hydrogen bonds. However, any impurities, like salts, in the water enable it to conduct electricity. To create a conduction band with freely moving electrons, water would have to be pressurized to such an extent that the orbitals of the outer electrons overlap, something that only exists deep inside of large planets such as Jupiter.
Now, a team of researchers from 11 institutions around the world have used a completely different approach to create metallic water for the first time. They have achieved that feat by forming a thin layer of gold-colored metallic water on the outside of a droplet of liquid metal and documented this phase transition at the BESSY II facility in Berlin.
The key to the breakthrough was to pair the water with alkali metals, which release their outer electron very easily.
The problem is that water and alkali metals are notoriously difficult to mix – metals can fizzle, ignite, and even explode when dropped into water. But, the researchers managed to avoid a potential explosion by not putting the metal into the water but rather by pouring a bit of water onto the alkali metal, a sodium-potassium (Na-K) alloy, which is liquid at room temperature.
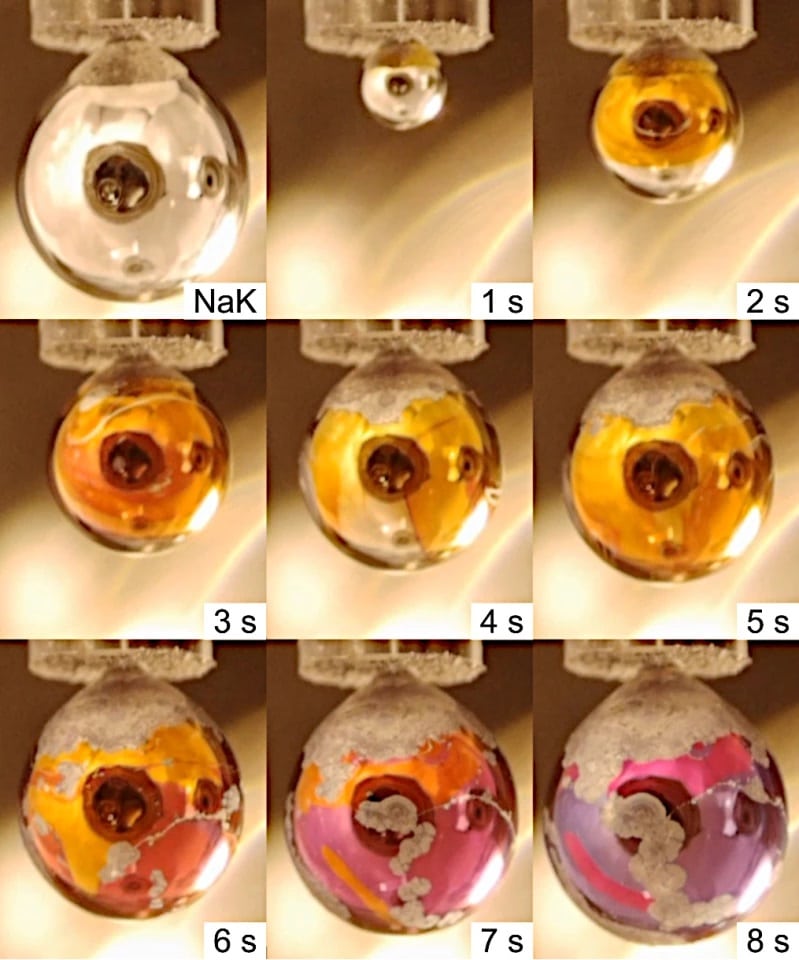
The experimental setup consists of a high vacuum sample chamber that contains a fine nozzle from which the liquid Na-K alloy drips. The silver droplet grows for about 10 seconds until it detaches from the nozzle. As the droplet grows, some water vapor flows into the sample chamber and forms an extremely thin skin on the surface of the droplet, only a few layers of water molecules. This almost immediately causes the electron and metal cations to dissolve from the alkali alloy into the water, creating conductive metallic water.
“You can see the phase transition to metallic water with the naked eye! The silvery sodium-potassium droplet covers itself with a golden glow, which is very impressive,” reports Dr. Robert Seidel, who supervised the experiments at BESSY II.
This setup leads to the formation of a transient gold-colored layer of a metallic water solution covering the metal alloy drops. The thin layer remains visible for a few seconds, enabling the researchers to prove with spectroscopic analyses at the BESSY II that it is indeed water in a metallic state.
“Our study not only shows that metallic water can indeed be produced on Earth but also characterizes the spectroscopic properties associated with its beautiful golden metallic luster,” says Seidel.
It still remains unclear what are the potential applications of such a technology.
