Lithium-metal-based solid-state batteries (Li-SSBs) are one of the most promising energy storage devices due to their high energy densities. These batteries are distinct from other batteries because they replace the flammable liquid electrolyte in conventional batteries with a solid electrolyte and use lithium metal as the anode. The use of solid electrolytes improves safety, and the use of lithium metal means more energy can be stored.
However, Li dendrites form on charging at practical rates and penetrate the ceramic electrolyte, leading to short circuits and battery failure.
A new study led by University of Oxford researchers has revealed mechanisms that cause lithium metal solid-state batteries to fail. If the challenges can be overcome, solid-state batteries using lithium metal anodes could deliver a step-change improvement in EV battery range, safety, and performance and help advance electrically powered aviation.
In their study, researchers used an advanced imaging technique called X-ray computed tomography at Diamond Light Source to visualize dendrite failure in unprecedented detail during the charging process. The advanced imaging techniques revealed that the initiation and propagation of the dendrite cracks are separate processes driven by distinct underlying mechanisms.
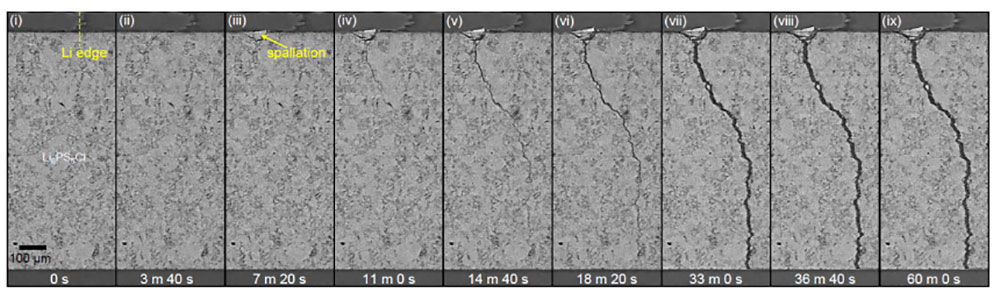
Dendrite cracks initiate when lithium accumulates in sub-surface pores. When the pores become full, further charging of the battery increases the pressure, leading to cracking. In contrast, propagation occurs with lithium only partially filling the crack through a wedge-opening mechanism which drives the crack open from the rear.
This new understanding points the way forward to overcome the technological challenges of Li-SSBs.
“For instance, while pressure at the lithium anode can be good to avoid gaps developing at the interface with the solid electrolyte on discharge, our results demonstrate that too much pressure can be detrimental, making dendrite propagation and short-circuit on charging more likely,” said Dominic Melvin, a Ph.D. student in the University of Oxford’s Department of Materials and one of the co-lead authors of the study.
“The process by which a soft metal such as lithium can penetrate a highly dense hard ceramic electrolyte has proved challenging to understand with many important contributions by excellent scientists around the world. We hope the additional insights we have gained will help the progress of solid-state battery research towards a practical device,” said Sir Peter Bruce, corresponding author of the study.
Journal reference:
- Ziyang Ning, Guanchen Li, Dominic L. R. Melvin, Yang Chen, Junfu Bu, Dominic Spencer-Jolly, Junliang Liu, Bingkun Hu, Xiangwen Gao, Johann Perera, Chen Gong, Shengda D. Pu, Shengming Zhang, Boyang Liu, Gareth O. Hartley, Andrew J. Bodey, Richard I. Todd, Patrick S. Grant, David E. J. Armstrong, T. James Marrow, Charles W. Monroe, and Peter G. Bruce. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature, 2023; DOI: 10.1038/s41586-023-05970-4
