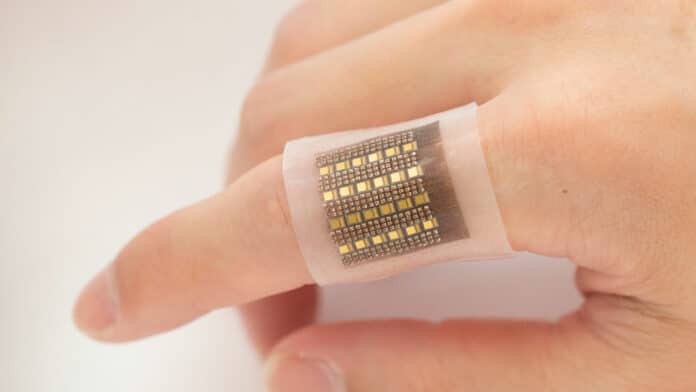
Engineers at the University of California San Diego have developed an electronic patch that can monitor biomolecules in deep tissues, including hemoglobin. The researchers’ idea is that, in the future, the patch will give medical professionals unprecedented access to crucial information that could help spot life-threatening conditions such as malignant tumors, organ dysfunction, cerebral or gut hemorrhages, and more.
Low blood perfusion inside the body may cause severe organ dysfunction, heart attacks, and vascular diseases of the extremities. Meanwhile, abnormal blood accumulation in areas such as in the brain, abdomen, or cysts can indicate cerebral or visceral hemorrhage or malignant tumors. Continuous monitoring can aid the diagnosis of these conditions and help facilitate timely and potentially life-saving interventions.
The new, flexible, low-form-factor wearable patch overcomes some significant limitations in existing methods of monitoring biomolecules. Existing technologies like MRI and X-ray-computed tomography rely on bulky equipment that can be hard to procure. Also, they usually only provide information on the immediate status of the molecule, making them unsuitable for long-term biomolecule monitoring.
With that in mind, the team’s patch is designed to offer a noninvasive long-term monitoring option for deep-lying biomolecules with the help of lasers. It can perform three-dimensional mapping of hemoglobin with a submillimeter spatial resolution in deep tissues, down to centimeters below the skin, compared to other devices that only detect biomolecules on the surface.
“The amount and location of hemoglobin in the body provide critical information about blood perfusion or accumulation in specific locations. Our device shows great potential in close monitoring of high-risk groups, enabling timely interventions at urgent moments,” said Sheng Xu, a professor of nanoengineering at UC San Diego and corresponding author of the study.
The wearable patch itself is flexible and comfortably attaches to the skin. It features arrays of laser diodes and piezoelectric transducers in its soft silicone polymer matrix, sending pulsed lasers into the tissues below. Biomolecules in the tissue absorb the optical energy and radiate acoustic waves into surrounding media.
“Piezoelectric transducers receive the acoustic waves, which are processed in an electrical system to reconstruct the spatial mapping of the wave-emitting biomolecules,” said Xiaoxiang Gao, a postdoctoral researcher in Xu’s lab and co-author of the study.
The next step is to further develop the device, including shrinking the backend controlling system to a portable-sized device for laser diode driving and data acquisition, greatly expanding its flexibility and potential clinical utility. They also plan to explore the wearable’s potential for core temperature monitoring.
“Continuous monitoring is critical for timely interventions to prevent life-threatening conditions from worsening quickly,” said Xiangjun Chen, a nanoengineering Ph.D. student in the Xu group and study co-author. “Wearable devices based on electrochemistry for biomolecule detection, not limited to hemoglobin, are good candidates for long-term wearable monitoring applications. However, the existing technologies only achieve the ability of skin-surface detection.”
With access to biomolecules in deep tissues, this technology adds unprecedented capabilities to wearable electronics and thus holds significant implications for various applications in both basic research and clinical practice.
Journal reference:
- Xiaoxiang Gao, Xiangjun Chen, Hongjie Hu, Xinyu Wang, Wentong Yue, Jing Mu, Zhiyuan Lou, Ruiqi Zhang, Keren Shi, Xue Chen, Muyang Lin, Baiyan Qi, Sai Zhou, Chengchangfeng Lu, Yue Gu, Xinyi Yang, Hong Ding, Yangzhi Zhu, Hao Huang, Yuxiang Ma, Mohan Li, Aditya Mishra, Joseph Wang, and Sheng Xu. A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nature Communications, 2022; DOI: 10.1038/s41467-022-35455-3