In the modern world, rechargeable batteries are the primary energy source of portable electronic devices (PEDs) and hold the key to guarantee their desired performance stability. So, scientists around the world are actively working in this direction.
Nanoengineers at the University of California San Diego, in collaboration with California-based company ZPower, have developed a flexible, rechargeable silver oxide-zinc battery. According to the researchers, the device has an areal energy density that is 5-10 times greater than state of the art.
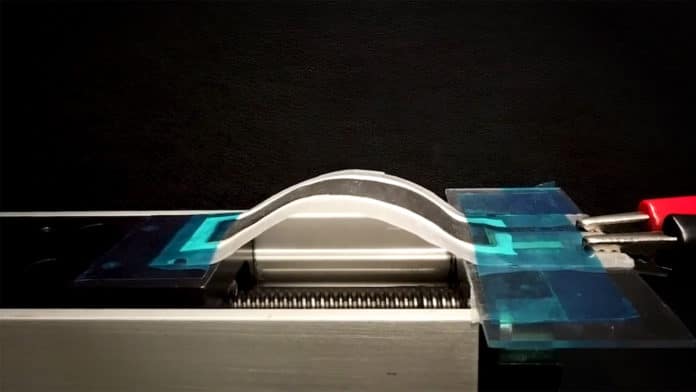
Manufactured by screen printing, these flexible battery consists is reportedly easier to manufacture than other flexible batteries. While the most flexible batteries need to be manufactured in sterile conditions, under vacuum, this one can be screen printed in normal lab conditions. It could be used in flexible, stretchable electronics for wearables and soft robots.
“Our batteries can be designed around electronics, instead of electronics needed to be designed around batteries,” said Lu Yin, a Ph.D. student in the research group of UC San Diego’s nanoengineering Professor Joseph Wang.
This innovative battery’s areal capacity is 50 milliamps per square centimeter at room temperature – this is 10-20 times greater than the areal capacity of a typical Lithium-ion battery. For the same surface area, the battery described in Joule can provide 5 to 10 times more power. In this case, compared to other batteries, the novelty from the developers from the United States boasts a significantly larger capacity.
“This kind of areal capacity has never been obtained before,” Yin said. “And our manufacturing method is affordable and scalable.“
The team said the battery’s exceptional energy density is due to its silver oxide-zinc (AgO-Zn) chemistry. Most commercial flexible batteries use Ag2O-Zn chemistry, which the researchers said limited cycle life and capacity. AgO is traditionally considered unstable, but ZPower’s AgO cathode material uses a proprietary lead oxide coating to improve its electrochemical stability and conductivity. The AgO-Zn chemistry is also responsible for the battery’s low impedance, while its printed current collectors have excellent conductivity, helping achieve lower impedance.
The batteries are printed on a polymer film resistant to a temperature of up to 200°C, and each element is screen printed on a separate layer. The battery can be printed onto a polymer film in “a few seconds” once the inks are prepared, and it is dry and ready to use in minutes. This process has the advantage of being usable under normal laboratory conditions, rather than a sterile and vacuum environment.
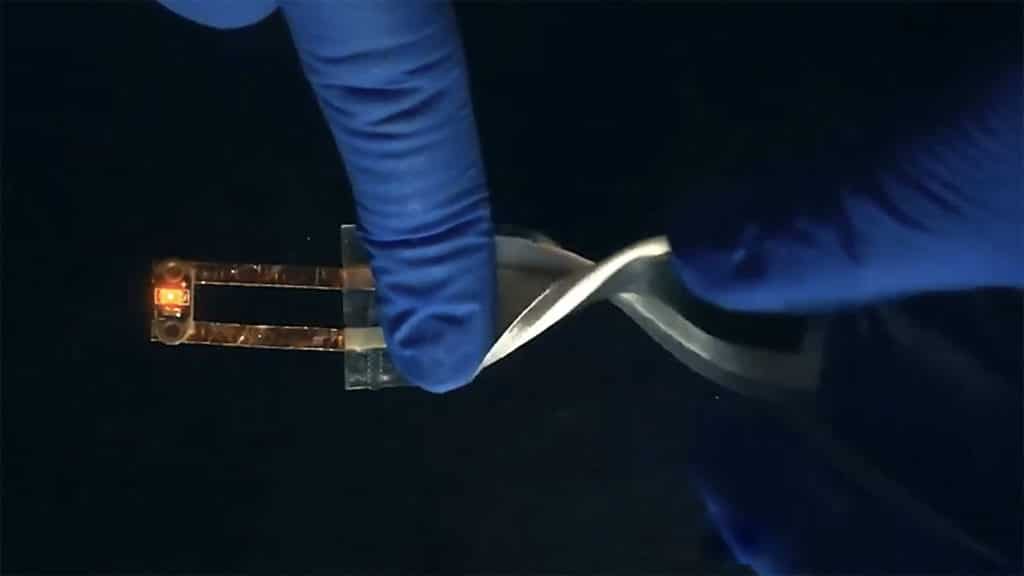
During the tests, the printed battery cells were recharged for more than 80 cycles without showing any major signs of capacity loss. The cells also remained functional despite repeated bending and twisting.
Researchers are already working on the next generation of this battery, which is expected to charge faster and cost less to produce. It is intended in particular for connected objects, new 5G devices, as well as soft robotics.