Many batteries contain liquid electrolytes, which makes them a potential fire hazard. However, all-solid-state lithium batteries employing inorganic solid electrolytes offer an enticing combination of higher safety and increased energy density and are exciting candidates for next-generation energy storage.
Now, researchers from the University of Waterloo, Canada, have discovered a new solid electrolyte that offers several important advantages. This electrolyte, composed of lithium, scandium, indium, and chlorine, conducts lithium ions well but electrons poorly. This combination is essential to creating an all-solid-state battery that functions without significantly losing the capacity for over a hundred cycles at high voltage (above 4 volts) and thousands of cycles at intermediate voltage.
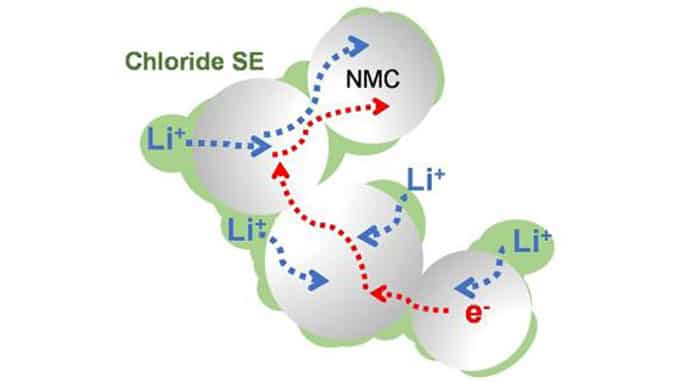
The chloride nature of the electrolyte is key to its stability at operating conditions above 4 volts, which means it is suitable for typical cathode materials that form the mainstay of today’s lithium-ion cells. “Chloride electrolytes have become increasingly attractive because they oxidize only at high voltages, and some are chemically compatible with the best cathodes we have,” said Linda Nazar, a Distinguished Research Professor of Chemistry at UWaterloo. “There’s been a few of them reported recently, but we designed one with distinct advantages.”
One chemical key to the ionic conductivity lay in the material‘s crisscrossing 3D structure called a spinel. The researchers had to balance two competing desires – to load the spinel with as many charge-carrying ions as possible but also to leave sites open for the ions to move through. They also needed to make sure that the electrons could not move easily through the electrolyte to trigger its decomposition at high voltage.
Nazar said that it is not yet clear why the electronic conductivity is lower than many previously reported chloride electrolytes, but it helps establish a clean interface between the cathode material and solid electrolyte, a fact that is largely responsible for the stable performance even with high amounts of active material in the cathode.
