The ever-growing demand for high-energy and low-cost batteries has boosted the exploration of novel electrochemistry beyond conventional lithium-ion batteries. Li-S batteries have drawn much attention because of their high theoretical energy density and the earth’s abundance of sulfur resources. However, from a practical perspective, these batteries exhibit poor cycle life and low energy content owing to the polysulfide shuttling during cycling.
In a new study, researchers at the U.S. Department of Energy’s (DOE) Argonne National Laboratory advanced sulfur-based battery research by creating a layer within the battery that adds energy storage capacity while nearly eliminating a traditional problem with sulfur batteries that caused corrosion. The promising battery design pairs a sulfur-containing positive electrode (cathode) with a lithium metal negative electrode (anode), and in between those is the electrolyte.
Initially, lithium-sulfur (Li-S) batteries did not perform well because sulfur species (polysulfides) dissolved into the electrolyte, causing its corrosion. This polysulfide shuttling effect negatively impacts battery life and lowers the number of times the battery can be recharged.
To prevent this polysulfide shuttling, previous researchers proposed the use of redox-inactive protective layers between the sulfur-containing cathode and lithium metal anode. However, this protective interlayer is heavy and dense, reducing energy storage capacity per unit weight for the battery.
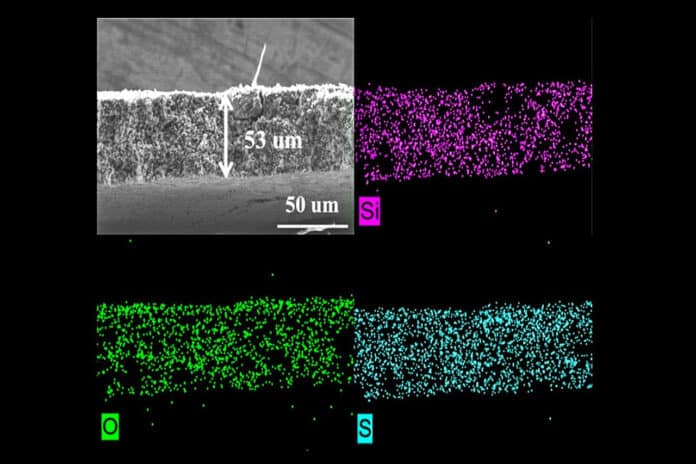
To solve this, researchers have now developed and tested a porous sulfur-containing redox-active interlayer. Differently from redox-inactive interlayers, these redox-active interlayers enable the electrochemical reactivation of the soluble polysulfides, protect the lithium metal electrode from detrimental reactions via silica-polysulfide polar-polar interactions and increase the cell capacity.
Lab tests showed initial capacity about three times higher in Li-S cells with this active, as opposed to inactive, interlayer. More impressively, the cells with the active interlayer maintained high capacity over 700 charge-discharge cycles.
To further study the redox-active layer, the team conducted experiments at the 17-BM beamline of Argonne’s Advanced Photon Source (APS), a DOE Office of Science user facility. The data gathered from exposing cells with this layer to X-ray beams confirmed that a redox-active interlayer could reduce shuttling, reduce detrimental reactions within the battery and increase the battery’s capacity to hold more charge and last for more cycles.
“These results demonstrate that a redox-active interlayer could have a huge impact on Li-S battery development,” said Wenqian Xu, a beamline scientist at APS. “We’re one step closer to seeing this technology in our everyday lives.”
Next, the team wants to evaluate the growth potential of the redox-active interlayer technology. “We want to try to make it much thinner, much lighter,” said Guiliang Xu, an Argonne chemist and co-author of the paper.
Journal reference:
- Byong-June Lee, Chen Zhao, Jeong-Hoon Yu, Tong-Hyun Kang, Hyean-Yeol Park, Joonhee Kang, Yongju Jung, Xiang Liu, Tianyi Li, Wenqian Xu, Xiao-Bing Zuo, Gui-Liang Xu, Khalil Amine and Jong-Sung Yu. Development of high-energy non-aqueous lithium-sulfur batteries via redox-active interlayer strategy. Nature Communications, 2022; DOI: 10.1038/s41467-022-31943-8