The huge amount of plastic waste that ends up in the form of microplastics in the oceans, soil, and drinking water, but also in human and animal bodies, is one of the main polluters of modern times. Scientists are always looking for new solutions to get rid of plastic waste as efficiently as possible.
One solution could be the use of cobalt-based catalysts. They would be able to break down mixed plastics into propane, which can then be burned as fuel or used to make new plastic.
A study by scientists from the Massachusetts Institute of Technology (MIT) points out that the main problem why the enormous amount of plastic waste cannot be simply disposed of today is its diversity. Plastics come in so many different varieties, and chemical processes for breaking them down into a form that can be reused in some way tend to be very specific to each type of plastic.
Waste sorting, which we consider today one of the primary methods of plastic recycling, is also inefficient. And much of the plastic material gathered through recycling programs end up in landfills anyway.
MIT team proposed a new chemical process using a catalyst that can deal with multiple plastics mixed together, converting them into a single product, propane. Propane can then be used as fuel for stoves, heaters, and vehicles or as a feedstock for the production of a wide variety of products.
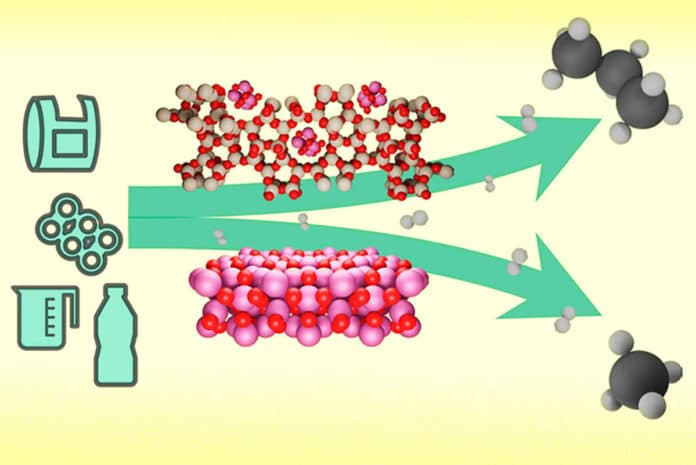
The new catalyst is made of a microporous material called zeolite that contains cobalt nanoparticles. Although zeolites are studded with tiny pores less than a nanometer in width, it was logical to assume that there would be little interaction between the zeolite and the polymers. Surprisingly, however, the opposite was shown: not only do the polymer chains enter the pores, but the synergistic work between cobalt and the acid sites in the zeolite can break the chain at the same point.
That cleavage site turned out to correspond to chopping off exactly one propane molecule without generating unwanted methane, leaving the rest of the longer hydrocarbons ready to undergo the process again and again. This works on a variety of plastics, such as polyethylene (PET) and polypropylene (PP).
The researchers tested their system on a real example of mixed recycled plastic, producing promising results. The process converted around 80% of the plastic into propane without producing methane as a by-product. The materials needed for the process, zeolites and cobalt, are both quite cheap and widely available.
In future work, researchers will need to focus on how the technique might be scaled for use in real-world plastic recycling streams and how it might be affected by contaminants like inks, glues, and labels attached to plastic containers.
Journal reference:
- Guido Zichittella, Amani M. Ebrahim, Jie Zhu, Anna E. Brenner, Griffin Drake, Gregg T. Beckham, Simon R. Bare, Julie E. Rorrer, and Yuriy Román-Leshkov. Hydrogenolysis of polyethylene and polypropylene into propane over cobalt-based catalysts. JACS Au 2022. DOI: 10.1021/jacsau.2c00402