Stabilizing atmospheric CO2 requires significant reductions in anthropogenic CO2 emissions. There have been extensive efforts to develop individual technologies for CO2 capture and CO2 utilization to curb carbon emissions efficiently. However, for rapid and economically viable reduction of atmospheric CO2, it is necessary to develop efficient technologies for integrated carbon capture and conversion to sustainably produce net-negative fuels in a closed carbon cycle.
Now, engineers at the University of Illinois Chicago have built an integrated, net-negative system that continuously captures carbon dioxide from flue gas and converts it to high-purity ethylene.
The first-of-its-kind technology integrates a carbon capture system with an ethylene conversation system. It runs on electricity but also removes more carbon from the environment than it generates – making it what scientists call net-negative on carbon emissions.
“This is the first demonstration of a net-negative, all-electric integrated system to capture carbon from pollutants and create a highly valuable resource,” said Meenesh Singh, UIC assistant professor in the department of chemical engineering. “This is an important milestone in ethylene decarbonization.”
Ethylene is a widely used chemical in plastic production, agriculture, the automotive industry, and more. Among manufactured chemicals worldwide, ethylene ranks third for carbon emissions after ammonia and cement.
To capture carbon from the air or flue gas, the researchers modified a standard artificial leaf system with inexpensive materials to include a water gradient – a dry side and a wet side – across an electrically charged membrane. The dry side included an organic solvent that attaches to available carbon dioxide to produce a concentration of bicarbonate, or baking soda, on the membrane. As the bicarbonate accumulates, these negatively charged ions are pulled across the membrane toward a positively charged electrode in a water-based solution on the membrane’s wet side. Subsequently, the liquid solution dissolves the bicarbonate back into carbon dioxide, so it can be released and harnessed for CO2 conversion.
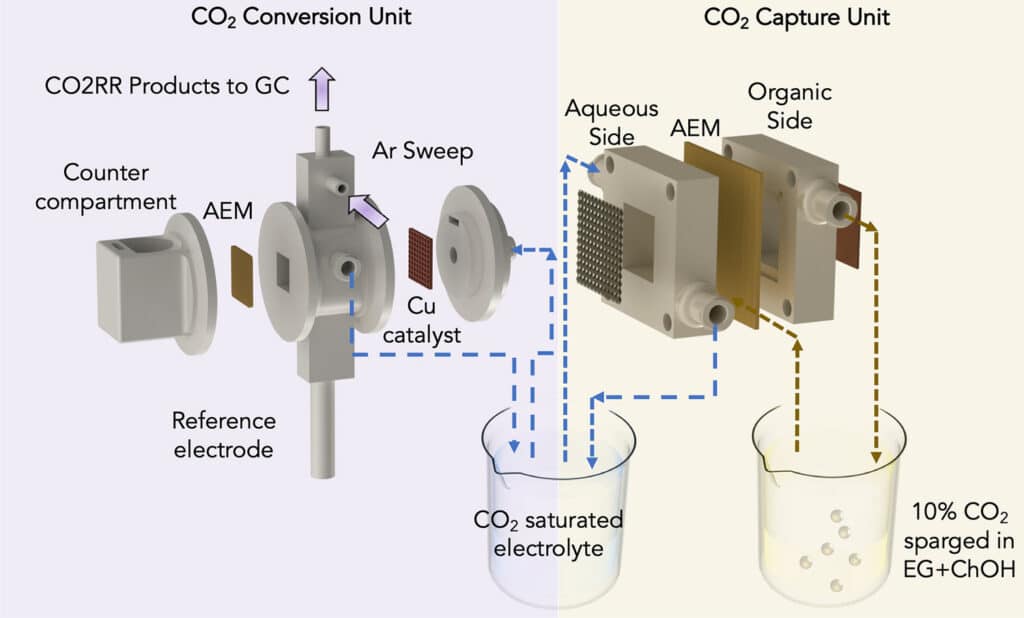
The UIC team employed a second system to convert captured carbon dioxide to ethylene, in which an electric current is passed through a cell. Half of the cell is filled with carbon dioxide captured from a carbon capture system, while the other half contains a water-based solution. An electrified catalyst draws charged hydrogen atoms from the water molecules into the other half of the unit, separated by a membrane, where they combine with charged carbon atoms from the carbon dioxide molecules to form ethylene.
Researchers integrated the two systems by feeding the captured carbon dioxide solution to the carbon conversion system and recycling it back. The closed-loop recycling process ensures a constant supply of carbon dioxide from flue gas and its conversion to ethylene.
The team tested their integrated system by implementing a 100-square-centimeters bipolar membrane electrodialysis unit to capture carbon dioxide from the flue gas and hydraulically connected it to the 1-square-centimeter electrolysis cell to produce ethylene. The system was tested continuously for one week, 24 hours per day. The system demonstrated exceptional stability and captured carbon at a rate of 24 grams daily, producing ethylene at 188 milligrams per day.
“In the journey to make ethylene production green, this is a potential breakthrough,” Singh said. “Our next step is to scale up the integrated carbon capture and conversion system to produce ethylene at higher rates – a rate of 1 kilogram per day and capture carbon at a rate higher than kilograms per day.”
Journal reference:
- Aditya Prajapati, Rohan Sartape, Miguel T. Galante, Jiahan Xie, Samuel L. Leung, Ivan Bessa, Marcio H. S. Andrade, Robert T. Somich, Márcio V. Rebouças, Gus T. Hutras, Nathália Dinizb and Meenesh R. Singh. Fully-integrated electrochemical system that captures CO2 from flue gas to produce value-added chemicals at ambient conditions. Energy & Environmental Science, 2022; DOI: 10.1039/D2EE03396H