Trains do not have the blinking turn signal lever found in automobiles. They do not have a manual handle to make a left or right turn. Instead, a train is governed by a switch point mechanism, which allows for controlled travel in a desired direction. This mode of operation can be likened to the functionality of a catalyst employed in fuel cells of hydrogen-powered vehicles to selectively trigger chemical reactions and impede corrosion.
A research team at POSTECH has developed a selective catalyst that curbs corrosion in fuel cells used for hydrogen-powered automobiles. By tailoring the hydrogen oxidation reaction to match the concentration of hydrogen in the fuel cell, the team could hinder the fuel cells’ corrosion.
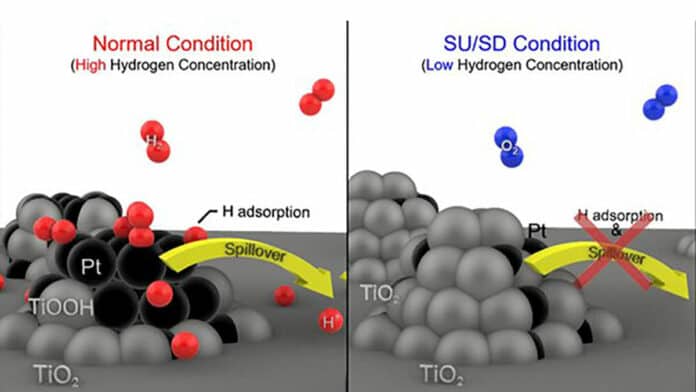
Researchers have engineered a catalyst (Pt/TiO2) comprising platinum (Pt) deposited onto a titanium dioxide (TiO2) that efficiently halts corrosion in fuel cells employed in hydrogen-powered automobiles. The performance of this electrocatalyst comes from the robust interaction between titanium dioxide and platinum and the ability of hydrogen spillover to modify the surface conductivity of the material in response to the hydrogen concentration in its vicinity.
When a vehicle suddenly stops or starts, the concentration of hydrogen within the fuel decreases correspondingly. This reduction in hydrogen concentration causes an expansion of titanium dioxide onto platinum, which results in platinum being buried beneath the catalyst’s surface.
Caused by the expansion of titanium dioxide, this burying of the platinum ultimately transforms the catalyst into an insulator due to the low conductivity of titanium dioxide. This insulating effect hinders the catalyst’s ability to conduct electricity, preventing an unwanted reduction of oxygen that could cause sudden potential jumps in the cathode.
In contrast, during a standard vehicle operation, the concentration of hydrogen within the car remains high. Under such high hydrogen concentration conditions, the highly conductive platinum is exposed on the catalyst’s surface, and titanium dioxide reduction occurs, which promotes hydrogen mobility on the catalyst’s surface. This phenomenon, called hydrogen spillover, enhances current flow and increases hydrogen oxidation reaction.
In the simulation test, the research team compared the newly developed catalyst and conventional catalysts. The test results demonstrated that fuel cells using Pt/TiO2 catalyst exhibited three times higher durability than traditional fuel cells. This indicated the team successfully increased fuel cells’ durability through a selective oxygen reduction reaction and a hydrogen oxidation reaction based on the hydrogen concentration.
If this research can contribute to overcoming the existing durability challenges confronting fuel cells for hydrogen-powered vehicles, then it could potentially elevate the standing of Korean hydrogen-fueled automobiles in the next-generation mobility industry.
Journal reference:
- Sang-Hoon You, Sang-Mun Jung, Kyu-Su Kim, Jinhyeon Lee, Jinkyu Park, Ho Yeon Jang, Sangyong Shin, Hyunjoo Lee, Seoin Back, Jinwoo Lee, and Yong-Tae Kim. Enhanced Durability of Automotive Fuel Cells via Selectivity Implementation by Hydrogen Spillover on the Electrocatalyst Surface. ACS Energy Letters, 2023. DOI: 10.1021/acsenergylett.2c02656