Since its first commercialization, Lithium-ion batteries (LIBs) have been widely used and are one of the most popular types of rechargeable battery for various electrochemical energy storage applications including mobile devices, electric vehicles, and energy storage system (ESS). But due to increasing demands, their prices have also gone up in the last few years.
As a promising alternative for LIBs, thus, sodium-ion batteries (SIBs) have been suggested owing to natural abundance and low cost of sodium, and its similar chemical nature to lithium. Despite their growing interest, commercialization of SIBs is far from realization due to the lack of suitable electrode materials.
Now, a team of researchers from the Korea Advanced Institute of Science and Technology (KAIST) presented a new strategy for extending the cyclability of sodium-ion batteries using copper sulfide as the electrode material. It could pave the way to high-performance conversion reactions of sodium-ion batteries (SIBs).
The research, led by Professor Jong Min Yuk, is also expected to forward the commercialization of SIBs as they emerge as an alternative to lithium-ion batteries.
Copper sulfide is suggested as a superior electrode material with high capacity, high rate, and long‐term cyclability owing to its unique conversion reaction mechanism that is pulverization‐tolerant and thus induces the capacity recovery.
The results of the research suggest that when employing copper sulfide, sodium-ion batteries will have a lifetime of more than five years with one charge per day. This work provides a key finding on high‐performance conversion reaction-based electrode materials for sodium-ion batteries. Because copper sulfide is made of abundant natural and low-cost material like copper and sulfur, it provides a better cost-effective alternative to the lithium-ion batteries.
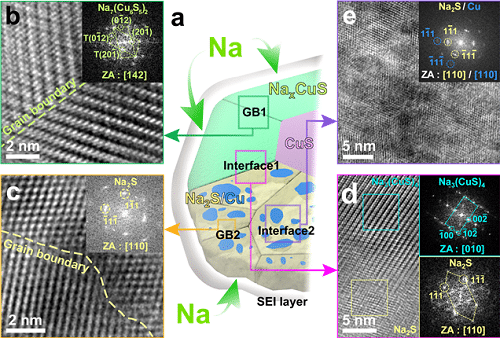
Lithium-ion batteries use intercalation-type materials such as graphite as an anode, which have not been viable for high-capacity sodium storage due to their insufficient interlayer spacing. Thus, conversion and alloying reactions type materials have been explored to meet higher capacity in the anode part. However, those materials generally bring large volume expansions and sudden crystallographic changes, which can lead to a decrease in efficiency.
Researchers confirmed that semi-coherent phase interfaces and grain boundaries in conversion reactions played an important role in enabling pulverization-tolerant conversion reactions and capacity recovery, respectively.
Read more: Indians teens invented a device that helps you charge your phone by walking
“Sodium-ion batteries employing copper sulfide can advance sodium-ion batteries, which could contribute to the development of low-cost energy storage systems and address the micro-dust issue,” said Professor Yuk.
Unlike intercalation reaction, conversion and alloying reactions generally involve abrupt crystallographic changes and huge volume expansion rate, which leads to capacity degradation by active materials destruction. However, copper sulfides were able to produce gradual crystallography for semi-coherent interfaces, which eventually prevented the pulverization of the particles.
Based on this unique mechanism, the team confirmed that copper sulfide shows high cycling stability and high capacity, regardless of its size and morphology.
This study is posted in Advanced Science.
