Lithium-sulfur batteries can hold much more energy than their Li-Ion counterparts, but they have some problems that make them unstable and also unusable after only a handful of charging cycles. There are a number of reports claiming several hundred cycles for these batteries, but it is achieved at the expense of other parameters – capacity, charging rate, resilience, and safety.
Now, a team of researchers at the University of Michigan has developed a biologically-inspired membrane that could quintuple the charge capacity of electric car batteries, thereby massively increasing their range. Researchers have shown that a network of aramid nanofibers recycled from Kevlar can enable lithium-sulfur batteries to overcome their Achilles heel of cycle life – the number of times it can be charged and discharged.
The membrane can isolate the lithium ions on the anode part from the polysulfides at the cathode. It can also prevent the dendrites that form on the anode from puncturing through to the cathode, a common cause of fires in traditional batteries.
Previously, the team had relied on networks of aramid nanofibers infused with an electrolyte gel to stop dendrites from reaching the cathode. But lithium-sulfur batteries have another problem: small molecules of lithium and sulfur form and flow to the lithium, attaching themselves and reducing the battery’s capacity.
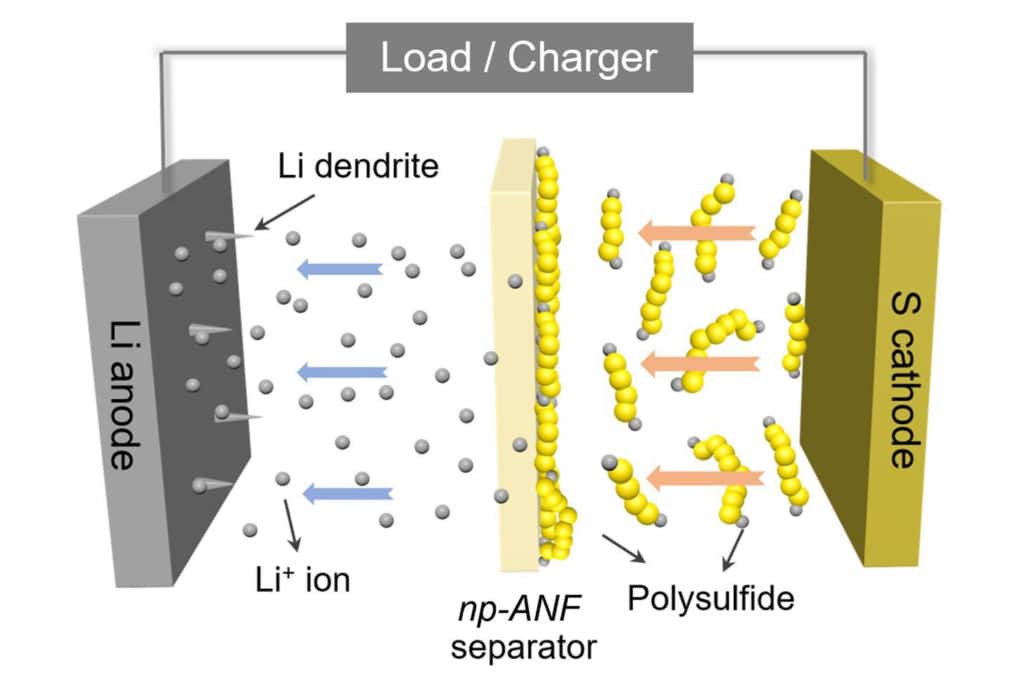
So to solve this problem, researchers developed a membrane that simultaneously allows lithium ions to flow from the lithium to the sulfur and back – and to block the lithium and sulfur particles, known as lithium polysulfides.
“Inspired by biological ion channels, we engineered highways for lithium ions where lithium polysulfides cannot pass the tolls,” said Ahmet Emre, a postdoctoral researcher in chemical engineering and co-first author of the paper.
The lithium ions and lithium polysulfides are similar in size, so it wasn’t enough to block the lithium polysulfides by making small channels. Mimicking pores in biological membranes, the U-M researchers added an electrical charge to the pores in the battery membrane. They stuck to the aramid nanofibers, and their negative charges repelled the lithium polysulfide ions that continued to form at the sulfur electrode. Positively charged lithium ions, however, could pass freely.
According to the researchers, the new design is nearly perfect as a battery, with its capacity and efficiency approaching the theoretical limits. It can also handle the temperature extremes of automotive life, from the heat of charging in full sun to the chill of winter. The team believes the breakthrough will enable real-world batteries to withstand more than 1,000 cycles under fast charging.
In addition to the higher capacity, lithium-sulfur batteries also have a number of sustainability advantages over other lithium-ion batteries. Sulfur is much more abundant than the cobalt of lithium-ion electrodes. Also, the aramid fibers of the battery membrane can be recycled from old bulletproof vests.
The University of Michigan has patented the membrane, and Nicholas Kotov, who led the research, is developing a company to bring it to market.
Journal reference:
- Mingqiang Wang, Ahmet E. Emre, Ji-Young Kim, Yiting Huang, Li Liu, Volkan Cecen, Yudong Huang, and Nicholas A. Kotov. Multifactorial engineering of biomimetic membranes for batteries with multiple high-performance parameters. Nature Communications, 2022; DOI: 10.1038/s41467-021-27861-w
